A research team led by Xu Xiaoling, associate professor in the Faculty of Health Sciences (FHS) of the University of Macau (UM), has made significant progress in its research on breast cancer metastasis. They used whole genome knockout library, and confirmed that ATP11b is the suppressor in breast cancer metastasis. The team identified the mechanism of ATP11b-mediated metastasis and the corresponding targeted therapeutic agents through cellular and animal model validation, which is expected to improve the treatment outcome for patients with metastatic breast cancer.The study has been published in the Journal of Clinical Investigation.
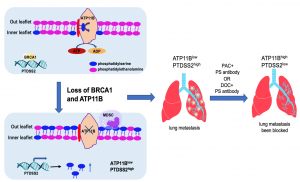
Tumour metastasis is the most common cause of death in breast cancer patients. Given the complicated process of metastasis, which includes abnormal proliferation, in situ invasion, vascular invasion, and colonization in distant organs, the role of the driver gene in the initiation of metastasis has attracted considerable attention. Previous studies mostly focus on abnormal gene expressions in the metastatic tumour using the next-generation sequencing (NGS), followed by biological validation to locate the relevant driver genes. Yet, only a few of them could rule out the interference of passenger genes detected by NGS. Moreover, BRCA1 mutant tumour is prone to metastasis, and blocking BRCA1-induced metastasis has been a challenge for researchers worldwide. Finally, there are few drugs for patients with metastatic cancer to choose from, so finding candidate drugs to change the expression of driver genes and helping patients choose suitable drugs have also become increasingly important.
The research team used genome-wide knockout library technology to screen the driver genes in whole genome-wide and identified the common metastasis suppressor ATP11b. Knocking out ATP11b in the DNA level significantly increases the ability of metastasis of BRCA1 mutant cells. Inducing non-apoptotic phosphatidylserine (PS) exposure on the cell membrane generates immune suppressive signal in tumour microenvironment (TME) and promotes the formation of pre-metastatic niche.The team confirmed that paclitaxel and doxorubicin were effective in blocking ATP11b-associated tumour metastasis through public database research and cellular validation.
The corresponding authors of the study are Prof Xu Xiaoling and Prof Deng Chuxia. The first author is PhD student Xu Jun. Other postgraduate students of FHS, namely PhD students Su Sek Man and Zhang Xin, and master’s student Chan Un In; research assistant Ragini Adhav; research assistant professor Miao Kai; as well as members of the animal facility also made important contributions to this study. The study was supported by the Science and Technology Development Fund (FDCT) grants (027/2015/A1, 029/2017/A1, 0101/2018/A3, 0112/2019/A2, 0011/2019/AKP, 0097/2021/A2, and 0045/2021/AFJ) and UM Multi-Year Research Grant (MYRG2016-00138-FHS, MYRG2017-00088-FHS, MYRG2019-00064-FHS). The full version of the research article can be viewed at: https://www.jci.org/articles/view/149473/pdf
| Source: Faculty of Health Sciences | |
| Media Contact Information: | |
| Communications Office, University of Macau | |
| Albee Lei | Tel: (853) 8822 8004 |
| Cathy Cheang | Tel: (853) 8822 8009 |
| Email: | prs.media@um.edu.mo |