A research team led by Dai Yunlu, associate professor in the Faculty of Health Sciences at the University of Macau (UM), has developed a novel nano-radiosensitizer capable of significantly improving the radiotherapeutic outcome of breast cancer. This nanomedicine not only improves the efficacy of radiotherapy but also relieves the MYC-correlated immunosuppression induced by radiation, thereby triggering a systemic anti-tumour immune response and effectively inhibiting the growth, recurrence, and metastasis of breast cancer. The research has been published in the internationally renowned journal Advanced Materials.
Although radiotherapy is clinically effective in inhibiting tumour growth, patients often suffer from severe immune tolerance and immunosuppression. One main reason is that cancer cells increase the expression of MYC protein after receiving the radiation. This protein possesses the capacity to repair DNA damage caused by radiotherapy, which promotes the proliferation of cancer cells and inhibits the type I interferon (IFN-I) signalling pathway. Impaired IFN-I signalling further impedes antigen presentation, disturbs the activation of cytotoxic T lymphocytes (CTLs), and triggers the expansion of tumour-associated regulatory T (Treg) cells. These cascade effects that MYC overexpression brings in highly inhibit the radiotherapeutic outcomes. Therefore, downregulating the MYC level of tumour cells may overcome the critical immunosuppressive barrier and elicit robust immuno-radiotherapy.
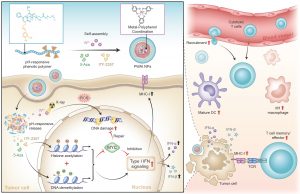
The research team designed an advanced nano-radiosensitizer (PWAI) to launch a dual-epigenetic reprogramming in radiotherapy. The PWAI can coordinate high-Z tungsten metal ions (W6+) with PEG-polyphenols by utilising a metal-phenolic network and load 5-Aza (a DNA methyltransferase inhibitor) and ITF-2357 (a histone deacetylase inhibitor) into the hydrophobic core.
Under the stimulation of exotic radiation, X-ray-sensitive W6+ efficiently destroyed the DNA strands of tumour cells by generating plentiful reactive oxygen species and meanwhile, two types of epigenetic inhibitors were released to modulate MYC dual-epigenetically. 5-Aza alone did not trigger IFN-α/IFN-β generation although it downregulated MYC protein. To combine 5-Aza and ITF-2357, apparent MYC inhibitory effect was still recognised in treated cancer cells; and more importantly, cellular levels of IFN-I signal-related proteins involving IFN-α, IFN-β, and the major histocompatibility complex I were significantly enhanced. To treat 4T1 tumour-bearing mice with PWAI nano-radiosensitizers, the research team identified the awakened anti-tumour immune response after radiotherapy, including the maturation of dendritic cells, cytotoxic T lymphocytes recruitment and their memory-phenotype formation, as well as the immune polarization of tumour-associated macrophages from immunosuppressive state to immunosupportive condition. Bringing dual-epigenetic reprogramming in radiotherapy may provide a potential strategy for advanced immuno-radiotherapy.
The corresponding authors of this paper are Dai Yunlu, and Li Bei, research assistant professor in the Faculty of Health Sciences at UM. PhD graduates Wang Guohao, Yan Jie, and Tian Hao are the co-first authors. Postdoctoral fellows Li Wenxi and PhD students Yu Xinying, Feng Yuzhao and Zhou Songtao also made important contributions to the study. The research project is funded by the Science and Technology Development Fund of the Macao SAR (File no: 0103/2021/A, 0002/2021/AKP, 0133/2022/A3, 0009/2022/AKP, and 0006/2023/ITP1), UM (File no: MYRG2022-00011-FHS and MYRG-GRG2023-00013-FHS-UMDF). The full text of the research paper is available at: https://onlinelibrary.wiley.com/doi/full/10.1002/adma.202312588
| Source: Faculty of Health Sciences | |
| Media Contact Information: | |
| Communications Office, University of Macau | |
| Albee Lei | Tel: (853) 8822 8004 |
| Cravina Chong | Tel: (853) 8822 4323 |
| Email: | prs.media@um.edu.mo |