Food poisoning from seafood remains a concern to many and there is ongoing research into the underlying causes. Our team has found that a toxin called RhsP, produced by the bacterium Vibrio parahaemolyticus, plays a key role in seafood food poisoning. We have studied the mechanism of this toxin and how it contributes to gastroenteritis by allowing the bacterium to compete for survival in the human gut. These findings lead to a deeper understanding of the cause of food poisoning and could be useful in developing new drugs and therapies.
RhsP: The Weapon of Vibrio Parahaemolyticus
Vibrio parahaemolyticus is a common bacterium found in coastal waters and seafood, especially shellfish such as scallops, oysters, and clams. The bacterium is most active in the summer and autumn months. It can enter the human intestine through the consumption of seafood or contact with seawater, where it multiplies and attacks neighbouring microorganisms.
The intestine harbours a variety of microorganisms, including a wide range of bacteria, which are collectively known as the gut flora. A sudden change in the balance of bacteria within the gut flora can lead to various health complications, such as impaired digestion, a weakened immune system, and an increased risk of chronic diseases.
How does V. parahaemolyticus attack other microorganisms after entering the gut? Our analysis of the V. parahaemolyticus genome has identified a protein toxin called RhsP. This protein, with a large structure of 1381 amino acids and a molecular weight of 157 kDa (a non-SI unit of mass used to express molecular mass, especially for large molecules such as proteins), is a polymorphic toxin that can change its structure. As a weapon, RhsP creates an environment that favours the proliferation of V. parahaemolyticus in the intestine, causing inflammation and damage to the intestinal mucosa with symptoms such as diarrhoea and vomiting.
Autoproteolysis, Dimerisation, and Attack
Our findings have shown that RhsP must first undergo a process known as autoproteolysis, which means breaking itself down into fragments. The fragmented RhsP then stick together in groups of two through a process known as dimerisation, forming RhsP dimers. The dimer favours the release of the C-terminal toxic (RhsPC), which is a nuclease that kills other bacteria in the gut by degrading their DNA.
How is this toxin transferred to other microorganisms? Our results show that V. parahaemolyticus delivers the RhsP toxin via its type 6 secretion system (T6SS). This system consists of several proteins that function together as a needle-like structure. For the C-terminal toxin RhsPC to reach its target, it needs to bind to one of the T6SS proteins, called VgrG2. One could compare V. parahaemolyticus to an army, with the T6SS serving as their cannons and the RhsPC toxin as the cannonballs.
The Self-Defense Mechanism of Vibrio Parahaemolyticus
To prevent committing suicide, V. parahaemolyticus produces an immunity protein called RhsPI to protect itself against its toxin. This RhsPI immunity protein binds to RhsPC and forms an RhsPC-RhsPI toxin-immunity pair to neutralise the toxicity by interacting with the active site of the RhsPC nuclease.
New Protein Structures Revealed
With the support of one of the most advanced third-generation synchrotron light sources in the world, the Shanghai Synchrotron Light Source of the Chinese Academy of Sciences (CAS), we used X-ray crystallography to determine the atomic structure of the RhsPC-RhsPI toxin-immunity pair and gain a detailed understanding of how the RhsPI immunity protein protects against the toxicity of RhsPC. This discovery marks the first case where X-ray crystallography has been used to determine a new complex protein structure by researchers based in Macao.
In addition, we collaborated with the team of Dr He Jun, a researcher at the Guangzhou Institute of Biomedicine and Health of the CAS, to use advanced cryo-electron microscopy techniques to reveal the structural changes and dimerisation of RhsP caused by autoproteolysis. This is also the first time that a Macao-based research institute has used cryo-electron microscopy to determine a new protein structure. These findings were published in the journal Cell Reports.
A Path to Unravel RhsP Toxin Delivery Mechanism
While our results have shown that RhsP dimerisation is a crucial step for V. parahaemolyticus to target its neighbours, the reason for this is still unclear. Further studies are needed to determine whether dimerisation occurs before RhsP is delivered via the T6SS or after the toxin has reached its target.
Author:William Chao is an associate professor in the Faculty of Health Sciences at UM. He holds a PhD from the Institute of Cancer Research in London, and an MBiochem from the University of Oxford. He applies structural biology techniques to study fundamental biological processes, particularly in the areas of cell division, chromatin structure, and epigenetics.
Text & Photo / William Chao
Chinese Translation / Davis Ip, UM Reporter John Leung
Source: UMagazine ISSUE 27
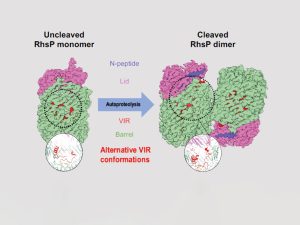
Structural comparison of RhsP before and after autoproteolysis
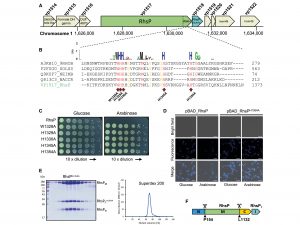
RhsP toxin autoproteolyses into three fragments

The crystal structure of the RhsPC-RhsPI toxin-immunity pair

Prof William Chao with his research team members
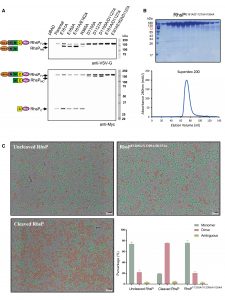
Autoproteolysis of RhsP toxin can promote its dimerisation, which is a necessary step for prey targeting
Prof William Chao
