Prof Ren-He Xu in the University of Macau (UM) Faculty of Health Sciences (FHS) and his research team have discovered a new way to enhance fat engraftment with the use of mesenchymal stromal cells (MSCs) derived from human embryonic stem cells (hESCs). This type of MSCs has the potential to be used as an innovative, cell-based biomaterial to support lipofilling widely used in cosmetic surgery. The related research finding has been published in the internationally renowned journal Biomaterials.
Autologous fat grafting has been widely used in cosmetic surgery for reconstructing facial and body parts after cancer surgery, in treating scars and wrinkles, and in breast enhancement. However, the graft survival rate varies dramatically between 25 per cent and 90 per cent because fat tissues become necrotic and fibrotic due to lack of blood supply. It has been known that MSCs isolated from somatic tissues such as adipose, bone marrow, and umbilical cord can increase the survival of transplanted tissues by promoting vascularisation and regeneration. However, somatic tissue-derived MSCs rely on donations and the process carries the risk of transmitting potential pathogens or genetically mutated cells. Although isolation of MSCs from autologous tissues is free of this problem, there is only a limited number of autologous MSCs available. In contrast, hESCs are an unlimited source with reliable and consistent quality and hESC-derived MSCs can be a better substitute for somatic MSCs.
Prof Xu’s team has demonstrated through multiple animal disease models that MSCs derived from hESCs via a trophoblast stage (T-MSCTM) have significant therapeutic effects in mice and/or monkeys with multiple sclerosis, colitis, and osteoarthritis. They also promote wound healing in both wild-type and diabetic mice. T-MSCs are patented in both the United States and China, and preclinical studies have been completed on their pharmacodynamics and biosafety in animals. Last year, the US Food and Drug Administration approved T-MSCs as an Investigational New Drug (IND) for clinical trials on multiple sclerosis.
In this study, Prof Xu’s team has found that T-MSCs enhance fat graft survival and retain graft weight comparable to the stromal vascular fraction (SVF) or MSCs derived from fat tissues which are the techniques currently used in clinics to improve fat engraftment. The team has also found that T-MSCs help glue dissociated fat tissues to form a capsulated graft within as early as 24 hours of transplantation and facilitate graft remodelling by forming new blood vessels and adipocyte regeneration. A patent application has been filed in both China and the US on the novel utilisation of T-MSCs.
Prof Xu is the corresponding author of the study entitled ‘Human ESC-derived MSCs Enhance Fat Engraftment by Promoting Adipocyte Reaggregation, Secreting CCL2 and Mobilizing Macrophages’. The study was led by Prof Xu, with the contributions of his PhD students Roma Borkar and Zheng Dejin, postdoctoral fellow Wang Xiaoyan, and research assistant Dr Li Enqin. Miao Zhengqiang, a student of FHS Associate Professor Chris Wong’s, provided bioinformatic analysis. This project was funded by Macau Science and Technology Development Fund (file number: 095/2017/A1 and 0112/2018/A3), UM Research Committee funds (file number: MYRG-2016-00070-FHS, MYRG-2017-00124-FHS, and CPG-2021-00031-FHS), and FDCT-National Natural Science Foundation of China joint grant (file number: 0008/2019/AFJ).
| Source: Faculty of Health Sciences | |
| Media Contact Information: | |
| Communications Office, University of Macau | |
| Albee Lei | Tel: (853) 8822 8004 |
| Judite Lam | Tel: (853) 8822 8022 |
| Email: | prs.media@um.edu.mo |
| UM 40th | |
| anniversary website: | 40.um.edu.mo |
| UM Website: | www.um.edu.mo |
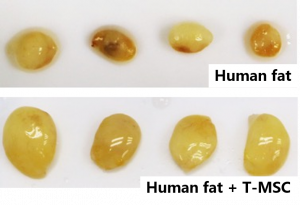
The use of MSCs enhances fat engraftment
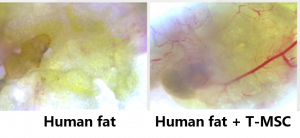
This type of MSCs has the potential to be used as an innovative, cell-based biomaterial to support lipofilling widely used in cosmetic surgery

Ren-He Xu (1st row, middle), Roma Borkar (3rd row, middle), Zheng Dejin (3rd row, 2nd from right), Wang Xiaoyan (3rd row, 3rd from left), and Li Enqin (1st row, 1st from left)
