A research team led by Xu Ren-He, Distinguished Professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), has made important progress in understanding the mechanisms of tumour immune evasion. They used stem cells to simulate mesenchymal stromal cells (MSCs) in the tumour microenvironment (TME), and found that MSCs can produce a cascade of intercellular signals by interacting with tumour cells, thereby enhancing the resistance of tumour cells against natural killer (NK) cells and promoting tumour metastasis. These findings, validated in clinical samples, reveal a novel mechanism of tumour immune evasion and provide a new therapeutic target for anti-tumour immunotherapy. The research findings have been published in the internationally renowned journal Advanced Science.
During tumour progression, cancer cells can evade the host immune system by recruiting inhibitory immune cells and secreting cytokines to form an immunosuppressive TME. Studies have demonstrated that MSCs, as an essential component of the TME, not only promote tumour cell proliferation, invasion, and metastasis, but also inhibit the functions of immune cells such as NK cells and T cells. Therefore, MSCs are considered to play a crucial role in tumour progression. However, there has been little research into the ability of MSCs to directly regulate tumour cell resistance to immune cells.
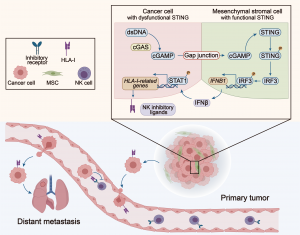
The research team observed that after coculturing with MSCs, the cancer cells exhibited an increased resistance to NK cell cytotoxicity. Further transcriptome analysis revealed that cocultured tumour cells expressed higher levels of NK inhibitory ligands, which helped the tumour cells escape NK cell-mediated cytotoxicity. It is noteworthy that this phenomenon is dependent on direct coculture between the cells. When MSCs and tumour cells were co-cultured indirectly or when gap junctions between the two cell types were blocked, the NK resistance disappeared. Further studies showed that abundant double-stranded DNA (dsDNA) is accumulated in the cytoplasm of tumour cells, which can be recognised by cGAS. cGAS then synthesises the second messenger cGAMP, which is transferred through gap junctions to surrounding MSCs, thereby activating the STING signalling pathway and inducing IFNβ production. IFNβ binds to tumour cells and upregulates their expression of NK inhibitory ligands, thereby enhancing tumour cell resistance to NK cells. In patients with breast cancer, non-small cell lung cancer and pancreatic cancer, expression levels of IFN receptors and the NK inhibitory ligands are significantly and negatively correlated with overall survival. Therefore, the study suggests that the interaction between tumour cells and MSCs, and the signalling pathway triggered by this interaction, may become a new target for tumour therapy.
The corresponding author of the paper is Prof Xu, and the first author is Yi Ye, a PhD student in FHS. FHS Dean Chuxia Deng, Professor Luo Qian, Associate Professor Liu Tzu-Ming, postdoctoral fellows Jia Hao, Yang Hongmei and Zeng Qibing, and PhD students Qin Guihui, Ye Sen, Zheng Dejin and Zhang Zhiming also made significant contributions to the study. All the core facilities of FHS, especially the Animal Research Core and the Biological Imaging and Stem Cell Core, provided substantial support for the research. The research project was supported by the National Key R&D Program of the Ministry of Science and Technology (File no: 2022YFA1105000), the National Natural Science Foundation of China (File no: 32270842), the Science and Technology Development Fund of the Macao SAR (File no: 0002-2021-AKP and 0071-2022-A2), and UM (File no: CPG2024-00037-FHS, MYRG2020-00140-FHS, and MYRG2022-00044-FHS). The full version of the research article is available at https://onlinelibrary.wiley.com/doi/10.1002/advs.202400888.
| Source: Faculty of Health Sciences | |
| Media Contact Information: | |
| Communications Office, University of Macau | |
| Albee Lei | Tel: (853) 8822 8004 |
| Jason Leong | Tel: (853) 8822 8322 |
| Email: | prs.media@um.edu.mo |