A research team led by Dai Yunlu, associate professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), has designed a novel nanomedicine, which can convert traditional radiotherapy-mediated apoptosis into pyroptosis, stimulate systemic anti-tumour immune response, and significantly inhibit breast cancer growth, recurrence, and metastasis. The research results have been published in the renowned journal Advanced Functional Materials.
Radiotherapy, although clinically effective for localised tumours, still cannot radio-functionalise them as a potent immunogenetic centre. This is because radiotherapy inhibits tumour cell growth by inducing apoptosis, such apoptotic nature limits its activating performance on systemic immunosurveillance. Apoptotic cancer cell maintains an intact membrane that conceals abundant potentially immunogenic damage-associated molecular patterns (DAMPs) inside, the releasing of whom, otherwise, could evoke surrounding immune cells remarkably.
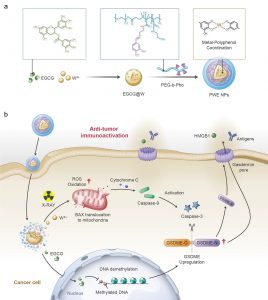
By W6+-coordinating natural polyphenols and chemically synthesised polyphenol derivatives, the research team developed a metal-polyphenolic nanocoordinator (PWE), which can epigenetically convert radiotherapy-mediated cell apoptosis to pyroptosis, thereby highly efficiently stimulating a systemic anti-tumour immune response. Specifically, PWE could be constructed via metal-phenolic coordination between poly-phenolic DNA methyltransferase (epigallocatechin-3-gallate, EGCG), high-Z radiosensitiser (W6+), and polyphenol-modified block copolymer. EGCG downregulated DNA hypermethylation of tumour cells and recovered their normal expression of the functional GSDME protein, a key protein of pyroptosis, while noble tungsten ions (W6+) sensitised by X-ray radiation boosted the production of cellular reactive oxygen species (ROS) that initiated the activation of caspase-3 through a potential pathway of Bax-Cytochrome c-caspase-9-caspase-3. The activated caspase-3 could be utilised to cleave GSDME upregulated by an EGCG demethylation, generating GSDME-N for cellular membrane perforation. The formed gasdermin pores enabled intracellular contents of GSDME, HMGB1, and lactate dehydrogenase (LDH) to pass through and release out of the cell, stimulating the immune system and reinvigorating anti-tumour immune responses. Furthermore, in treating 4T1 orthotopic breast tumour-bearing mice with the PWE nanomudulators, the research team identified efficient anti-tumour immune activity, long-lasting immunological memory, and metastatic inhibition. The nanocoordinator developed by metal-polyphenol coordination reshapes and ameliorates tumour immunity in traditional radiotherapy significantly through epigenetic pyroptosis, which may provide new insights into clinical radiotherapy.
The corresponding author of the study is Prof Dai. FHS PhD graduate Wang Guohao and Research Assistant Professor Li Bei are the co-first authors. Postdoctoral fellows Xie Lisi and Li Jie, PhD graduates Sang Wei and Zhang Zhan, PhD students Tian Hao, Yan Jie, and Li Wenxi also made important contributions to the study. The research project was supported by the Science and Technology Development Fund of the Macao SAR (File no: 0109/2018/A3, 0011/2019/AKP, 0113/2019/A2, 0103/2021/A, and 0002/2021/AKP), UM (File no: MYRG2022-00011-FHS), and the Shenzhen-Hong Kong-Macao Science and Technology Innovation Project (Category C) (File no: SGDX20201103093600004). The full version of the research article can be viewed at https://onlinelibrary.wiley.com/doi/10.1002/adfm.202213425.
| Source: Faculty of Health Sciences | |
| Media Contact Information: | |
| Communications Office, University of Macau | |
| Albee Lei | Tel: (853) 8822 8004 |
| Bell Leong | Tel: (853) 8822 8009 |
| Email: | prs.media@um.edu.mo |