A team led by Yunlu Dai, the associate professor in the Faculty of Health Sciences (FHS) of the University of Macau (UM) has made a significant breakthrough in postoperative cancer therapy in a research collaboration with a team led by Chu-Xia Deng, dean of the FHS, UM. The research teams developed a flex-patch to fuel metastatic sentinel lymph nodes (SLNs), activating tumour antigen-specific CD8+ T cells and their memory subsets to facilitate long-term tumour-specific immune protection. The study has received considerable attention in the field and has been published in the international journal Nature Communications.
The study confirms the positive role of tumour-infiltrating SLNs in postoperative immune regulation, resolving the clinical debate over whether lymph nodes must be surgically removed. In recent years, an increasing number of clinical researchers have questioned the necessity of removing SLNs during surgery. For example, in breast cancer, after tracking a large number of patients with existing SLN metastasis, it was found that the removal of axillary lymph nodes had little impact on the disease-free survival of surgical patients. Furthermore, as the first lymph nodes to receive tumor drainage, SLNs contain a rich population of immune cells that receive stimulation from tumour antigens and are crucial for the production of tumour-specific anti-tumour T cells. However, these T cells are susceptible to immune suppression by invading cancer cells, presenting a dilemma in the fight against cancer. Exploring the immune suppression status of T cells in SLNs and specifically activating their anti-tumour immune function is of great significance for optimising clinical surgical procedures and improving postoperative patient recovery.
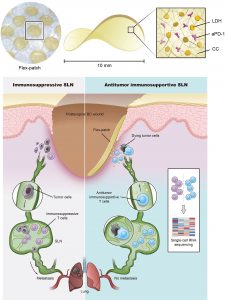
The team leveraged multiple analytic methods, including single-cell RNA sequencing and glycolytic analysis, to study metastatic SLNs from the orthotopic 4T1 breast tumour model. In addition to expressing PD-1, activated CD8+ T cells from metastatic SLNs enriched the citric acid cycle and oxidative phosphorylation pathways and did not meet the bioenergetic requirements per se for killing. Instead, they primarily relied on aerobic respiration to maintain their basic energy needs. Hence, the teams developed a biologically safe and immune-fueling flex-patch for SLN reshaping. After the surgical removal of the tumour and retention of the SLN, a flex-patch was implanted on the postoperative wound (Figure 1). The patch can slowly release the immunotherapeutic anti-PD-1 antibody (aPD-1) and nanoadjuvants (magnesium iron-layered double hydroxide, LDH) by responding to the surrounding microenvironment. Through the lymphatic and blood vessels between the tumor and SLN, both types of treatment agents can cross spatial and temporal barriers to reach the SLN. The therapeutic antibody targets PD-1 on the surface of activated CD8+ T cells, which can upregulate their glycolysis activity and tumour killing ability but has no effect on inactive CD8+ T cells. LDH nanoadjuvants, different from aPD-1, comprehensively promoted CD8+ T cell effector function (e.g. activation and cytotoxic killing) via Mg2+/Fe3+-mediated shaping. Ultimately, the levels of tumour antigen-specific CD8+ T cells in patch-driven metastatic SLNs were significantly upregulated, improving postsurgical immunoadjuvant therapy. This flex-patch bridges the gap between SLN immunoreshaping and postsurgical treatment.
It is worth noting that the biocompatible chitosan derivative (CC in Figure 1) was selected as the flex-patch matrix, ensuring that the regulation of SLN immune efficacy would not affect postoperative wound healing. The patch is easy to use and can be directly attached to the wound after tumour removal, without causing secondary harm to the patient. In addition, the treatment components carried by the patch can be flexibly adjusted, which is expected to promote the innovation of personalised postoperative adjuvant therapy.
Prof Dai and Prof Deng are the corresponding authors of the study. FHS Research Assistant Professor Li Bei, Assistant Professor Miao Kai, and PhD graduate Wang Guohao share the co-first authorship. Others who have contributed to the study include postdoctoral fellows Lei Haipeng, Xie Lisi, and Yan Jie, PhD student Zhang Aiping, as well as FHS research assistants Sun Liangyu, Yu Xinwang, and Li Wenxi. The Proteomics, Metabolomics and Drug Development Core, Animal Research Core and Biological Imaging and Stem Cell Core of FHS have also provided tremendous support for the study. This project was supported by the National Natural Science Foundation of China (File no: NSFC 32222090, 32101069 and 32171318), UM (File no: MYRG2022-00011-FHS), the Science and Technology Development Fund, Macao SAR (File no: 0109/2018/A3, 0011/2019/AKP, 0113/2019/A2, 0103/2021/A, 0092/2020/AMJ, and 0002/2021/AKP), Shenzhen-Hong Kong-Macau Science and Technology Programme Plan C (File no: SGDX20201103093600004 and SGDX20201103092601008), and Dr Stanley Ho Medical Development Foundation (SHMDF-OIRFS/2022/002). The full version of the research article can be viewed at https://www.nature.com/articles/s41467-023-38245-7.
| Source: Faculty of Health Sciences | |
| Media Contact Information: | |
| Communications Office, University of Macau | |
| Albee Lei | Tel: (853) 8822 8004 |
| Bell Leong | Tel: (853) 8822 8009 |
| Email: | prs.media@um.edu.mo |