A research team in the University of Macau (UM) Faculty of Health Sciences (FHS), led by Associate Professor Liu Tzu-Ming, has developed a new phosphorescence imaging method to map tumour hypoxia, which can help to precisely detect and remove cancerous tumours. This is an interdisciplinary collaboration between Prof Liu’s team and Prof Sergey P Tunik at Saint Petersburg State University, Prof Igor O Koshevoy at the University of Eastern Finland, and Prof Chou Pi-Tai at Taiwan University. The research team synthesised a new class of rhenium (ReI) diimine carbonyl complexes that can penetrate vessel walls after bolus injections. Then, the extravasated probes functionalise the collagen as an optically measurable tissue oxygen metre. It can help surgeons to identify satellite tumours commonly neglected in medical imaging. The study has been published in the prestigious journal Advanced Science.
Many unrecognisable seeds of tumours surround the major foci and create hypoxic environments locally. Tissue hypoxia may transform tumour biology, which can lead to drug resistance, cancer cell stemness, invasion, metastasis, dormancy, and enhanced clonal heterogeneity. These sites are hardly identified and removed in surgery. Therefore, detecting problematic hypoxia in vivo has remained a hot topic in biomedicine. Conventionally, the measurement of tissue oxygen levels relies on O2 microelectrodes. Despite the accuracy and immediacy, invasive insertion of an electrode stimulates the organ and inevitably causes metabolic disorders. That artefact may change the physiological status and the actual read-out of the O2 level. Besides, the lack of spatial information limits the use to navigate O2 distribution in vivo.
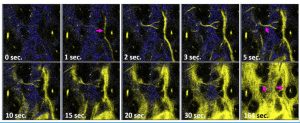
In this study, the team designed a new class of hydrophilic ReI-diimine luminescent complexes using water-soluble phosphine ligands and collagen-targeting sulfonic groups. Dissolved in water, the complex has an excellent emission yield of 40 per cent and a hypoxia phosphorescence lifetime τ of 4.0 µs. Without encapsulation or dendrimer protection, this probe can penetrate the vessel walls after venous administration. Then the probes are stabilised by collagen-binding and exhibited improved emission yield. The probes can stay in tissues and continuously monitor the oxygen level for more than six hours. In the centre of the tumour microenvironment, the probes measured an oxygen saturation level of 1 per cent (τ = 7.32 μs). At the site 5 mm away from the tumour, oxygen concentration was restored to a normal level (τ = 6.7 μs). Several in vivo studies in this work have shown that the phosphorescence probe can sense hypoxia in the tumour microenvironment and ischemia in tissues. With this vessel penetrating phosphorescence probes of oxygen, the sites with tumour hypoxia can be visualised, located, and precisely removed.
The study was led by Prof Liu, and the phosphorescence lifetime imaging of oxygen levels was conducted by PhD student Zhang Zhiming in the FHS. This project is supported by the Science and Technology Development Fund, Macau SAR (file number: 122/2016/A3, 018/2017/A1, 0011/2019/AKP and 0120/2020/A3) and UM’s research fund (file numbers: MYRG2018-00070-FHS and MYRG2019-00022-FHS). The full text of the research article can be viewed at: https://onlinelibrary.wiley.com/doi/full/10.1002/advs.202102788
| Source: Faculty of Health Sciences | |
| Media Contact Information: | |
| Communications Office, University of Macau | |
| Albee Lei | Tel: (853) 8822 8004 |
| Judite Lam | Tel: (853) 8822 8022 |
| Email: | prs.media@um.edu.mo |
| UM 40th | |
| anniversary website: | 40.um.edu.mo |
| UM Website: | www.um.edu.mo |