A team led by Dai Yunlu, associate professor in the Faculty of Health Sciences (FHS) of the University of Macau (UM), has made a breakthrough in developing magnesium-phenolic coordination nanoparticles that can slow the progression of brain tumours in the treatment of malignant glioblastoma. The study provides new insights into nanomedicine for the treatment of glioblastoma and lays the experimental groundwork for the clinical application of metalloimmunotherapy in the treatment of brain tumours. The research has been published in the internationally renowned journal ACS Nano.
Glioblastoma is an aggressive and malignant grade IV brain tumour that arises from astrocytes in the central nervous system and has a poor clinical response to emerging immunotherapies. At least 30% of the glioblastoma microenvironment (GME) is occupied by M2-like immunosuppressive macrophages that recruit regulatory T-cells and cause cytotoxic T lymphocyte (CTL) dysfunction. Repolarisation of M2 immunosuppressive macrophages to the proinflammatory M1 phenotype has been considered a fundamental strategy to address GME immune resistance. However, glioblastoma can exploit the immune tolerance behaviour of the organism to induce low T-cell receptor (TCR) affinity and high immunoinhibitory marker expression for CTL unresponsiveness, leading to CTL dysfunction. Therefore, regulating the function of gliomatous CTL is critical in the fight against brain tumours.
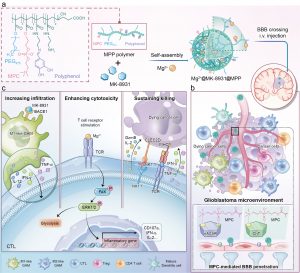
Previous studies have shown that metalloimmunology has powerful abilities to modulate T-cells against cancer. However, due to the complex physiological characteristics of the brain, most metal ions (such as the widely studied Mn2+, Zn2+, and Cu2+) carry the risk of inducing neurodegenerative diseases, limiting the application of metalloimmunology in the treatment of glioblastoma. Unlike other metal ions, magnesium ions (Mg2+) can sensitise the cGAS-STING pathway to trigger T-cell activation. Mg2+ supplementation can protect neural function and maintain brain health. Further studies have indicated that Mg2+ can reshape T-cell effector function by stimulating TCR, its downstream focal adhesion kinase (FAK), and extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) signals. Therefore, the use of Mg2+ as a cenogenetic immune adjuvant to enhance the functionality of incoming CTLs recruited by M1-like i-GAMs is expected to redress the gliomatous CTL sparsity and cytotoxic dysfunction at the same time.
Building on previous research, the research team synthesised an amphiphilic polyethylene glycol (PEG) polymer consisting of the choline analogue 2-methacryloyloxyethyl phosphorylcholine (MPC) and pyrocatechol moieties to coordinate Mg2+ and encapsulate the macrophage-repolarizing BACE1 inhibitor (MK-8931). MPC units ensured sufficient intra-glioblastoma accumulation. Next, pH-responsive release of MK-8931 repolarised macrophages to promote gliomatous CTL infiltration. Mg2+ then remodelled the cytotoxicity of gliomatous CTLs. In addition, CTL killing was further sustained by additional blocking with anti-NK1.1 antibodies. The nanoeditor was validated in vitro and in vivo, significantly optimising CTL functionality and prolonging the survival of mice bearing brain tumours. The promising data has broadened the horizon of glioblastoma treatment towards precise metalloimmunotherapy, laying the foundation for further clinical treatment of brain tumours.
The corresponding authors of the study are Prof Dai and Li Bei, research assistant professor in UM FHS. The co-first authors are Li Wenxi, a postdoctoral fellow, and Tian Hao, a PhD graduate of UM FHS. Yan Ziliang and Yu Xinying, PhD students of UM FHS, as well as members of the Biological Imaging and Stem Cell Core, Genomics, Bioinformatics and Single Cell Analysis Core, and Animal Research Core of UM FHS, also contributed to the study. The research was supported by the National Natural Science Foundation of China (File no.: 32222090, 32171318, and 32101069), UM (File no.: MYRG2022-00011-FHS), the University of Macau Development Foundation (File no.: MYRG-GRG2023-00013-FHS-UMDF), the Ministry of Education Frontiers Science Center for Precision Oncology, UM (File no.: SP2023-00001-FSCPO), and the Science and Technology Development Fund, Macao SAR (File no.: 0103/2021/A, 0002/2021/AKP, 0133/2022/A3, 0009/2022/AKP, and 0006/2023/ITP1). The full text of the research article is available at https://pubs.acs.org/doi/full/10.1021/acsnano.4c13388.
| Source: Faculty of Health Sciences | |
| Media Contact Information: | |
| Communications Office, University of Macau | |
| Albee Lei | Tel: (853) 8822 8004 |
| Bell Leong | Tel: (853) 8822 8009 |
| Email: | prs.media@um.edu.mo |