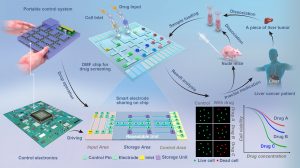
A research team led by Jia Yanwei, assistant professor in the Institute of Microelectronics at the University of Macau (UM), has developed a microfluidic system for drug screening using primary tumour cells, which can offer individual cancer patients a tailored treatment plan that exhibits the best therapeutic results. The system is expected to be used by clinicians in medication guidance, thereby realising the commercialisation of research results and facilitating technological innovation in the Guangdong-Hong Kong-Macao Greater Bay Area. The research findings have been published in the internationally renowned journal Nature Communications.
In recent years, precision medicine has emerged as a hot topic in the medical field. It represents a model of individualised healthcare that tailors the treatment plan and clinical decision for each patient based on the patient’s intrinsic biological information or clinical signs and symptoms. To date, the majority of precision medicine approaches have been based on the genetic abnormalities of each patient. Some drugs are prescribed to patients with certain genetic mutations with the aim of achieving an optimal response, whereas some other patients with specific mutations are not prescribed these drugs due to predicted reduced responsiveness or a high risk of adverse effects. However, clinical data have indicated that an increasing number of genes are involved in the cancer response to a certain drug, making the therapeutic effect based on genetic precision medicine unsatisfying. An alternative approach is to perform drug screening on primary tumour cells derived from patient biopsies or tumour resection samples. This provides direct information on the drug susceptibility of the specific tumour. However, biopsy samples contain only a limited number of cells, which makes drug screening with traditional 96-well microplates challenging. While multiple biopsies could provide sufficient tumour cells, this also carries an increased risk of cancer metastasis and causes the patient to experience more pain.
The research team has therefore developed a novel clinician-friendly drug screening device that enables the immediate collection of highly active cancer cells and on-site drug screening. It is a portable digital microfluidic (DMF) device with low power consumption that integrates all the controls into a handheld box with user-friendly control panels, enabling the handling of tiny samples. A single biopsy of a small quantity of cell samples is sufficient to produce drug screening results with the DMF chip, thus avoiding the risks and discomfort associated with repeated biopsies for patients. The device has been applied to clinical liver cancer samples for targeted drug screening. The results obtained are consistent with those derived from genetic sequencing and provide a more direct reflection of the individual patient’s response to the drug.
According to the research team, the DMF technology can provide clinicians and patients with reliable medication guidance, and low cost of equipment in comparison with that of gene sequencing. The technology should enable mass production and the realisation of precision medicine for all. It also provides researchers with a platform for high-throughput drug screening and significant savings in samples and reagents, which can help explore new therapeutic strategies and develop new drugs to further improve treatment efficacy. In addition, the DMF technology has been commercialised, resulting in the establishment of a high-tech service organisation called Promedicine Technology, which specialises in the research, development, production, and retail of precision medical equipment, contributing to technological innovation in the Greater Bay Area.
The corresponding author of the paper is Prof Jia, while UM postdoctoral fellow Zhai Jiao and UM doctoral student Liu Yingying are the co-first authors. Yao Hailong, professor at the University of Science and Technology Beijing, and Yi Shuhong, chief physician at the Third Affiliated Hospital of Sun Yat-Sen University, are the co-corresponding authors. UM professors Rui Martins and Mak Pui In, and the technical and management team of the State Key Laboratory of Analog and Mixed-Signal VLSI also made significant contributions to the study.
The research project was supported by the Science and Technology Development Fund of the Macao SAR (File no: 0029/2021/A1 and 004/2023/SKL), UM (File no: MYRG2020-00078-IME and MYRG-GRG2023-00034-IME), Dr Stanley Ho Medical Development Foundation (File no: SHMDF-OIRFS/2024/001), Zhuhai Huafa Group (File no: HF-006-2021), the Key Program of the National Natural Science Foundation of China (File no: 62034005), and the National Natural Science Foundation of China (File no: 61974084). The full version of the research article is available at https://www.nature.com/articles/s41467-024-48616-3.
| Source: Institute of Microelectronics | |
| Media Contact Information: | |
| Communications Office, University of Macau | |
| Albee Lei | Tel: (853) 8822 8004 |
| Jason Leong | Tel: (853) 8822 8322 |
| Email: | prs.media@um.edu.mo |